|
■
Home ■ site map |
|
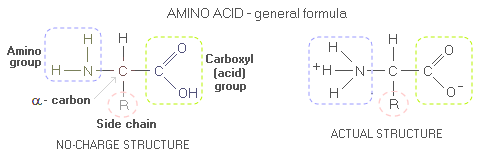
Body proteinBody protein - Daily requirement - Indispensable amino acids - Protein quality - PDCAAS - Optimum intake After water, body protein is its second most plentiful constituent-nutrient. Composed of amino acids, proteins are body's main tissue-building material, as well as a source of building material for body's metabolic and regulatory substances - enzymes, hormones, antibodies, DNA, RNA, and others. Usually regarded as the most important basic nutrient, proteins are, again, second only to water from the standpoint of body's most immediate nutritional necessities. When Dr. William Rose pioneered protein research on humans back in early 1950s, he has witnessed study participants suffering extreme fatigue, irritability and loss of appetite after as little as two days on insufficient intake of essential amino acids. During digestion, protein is broken down into its building blocks - amino acids - which the body uses to build thousands and thousands of its own proteins. The body needs 20 amino acids commonly found in living organisms); 8 of them that it cannot synthesize are called essential, or indispensable amino acids - Isoleucine, Leucine, Lysine, Methionine, Phenylalanine, Threonine, Tryptophan and Valine (with Histidine also being essential for infants and, possibly, semi-essential for adults too). The body has to obtain them from food as preformed amino acids. Secondary body function of proteins is as a source of energy. Each gram of protein burned for energy releases 4 calories. However, proteins are both, harder for the body to burn as well as needed for synthesis, and it normally satisfies less than 10% of its caloric needs by burning proteins. It will burn more proteins only when its preferred energy source - carbohydrates and fats - are not available. For molecularly curious, here's what protein molecule looks like. Proteins are complex molecules made of one of more polypeptide chains. Amino acids, that peptides are build from (peptide being generally a shorter chain of amino acids than polypeptide, usually assumed as having less than ~30 amino acids; technically, dipeptide - two amino acids joined together by peptide bond - is already polypeptide, and tripeptides like glutathione even more so), like all organic molecules, consists mainly of carbon and hydrogen. However, amino acid molecule also contain nitrogen atom, around which is built its amino group. The carbon atom linking amino group, acid group and amino acid's side chain (sub-molecular structure that varies from one amino acid to another, marked by R, for radical or rest) is called α-carbon. General form of an amino acid is often given as if without internal charge, i.e. with the amino and carboxyl (acid) groups as NH2 and COOH, respectively. In the actual amino acid molecule, the acidic COOH group reacts with the alkaline NH2 group, and the two transform into COO- and NH3+, respectively (the molecule as a whole still has no net charge).
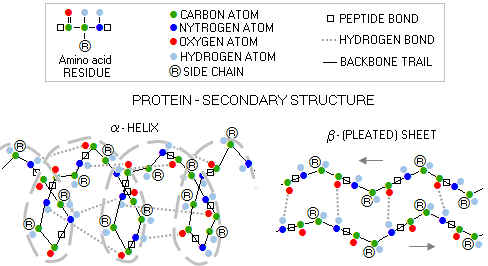
Peptide is built by amino acids bonding to each other
with - what else? - peptide bonds. In effect, two amino acid form
peptide bond when a water molecule is extracted from them by the enzyme
action. Slightly altered amino acids linked in the peptide chain are
called residues; the unaltered amino group on one end of (poly)peptide
chain is called
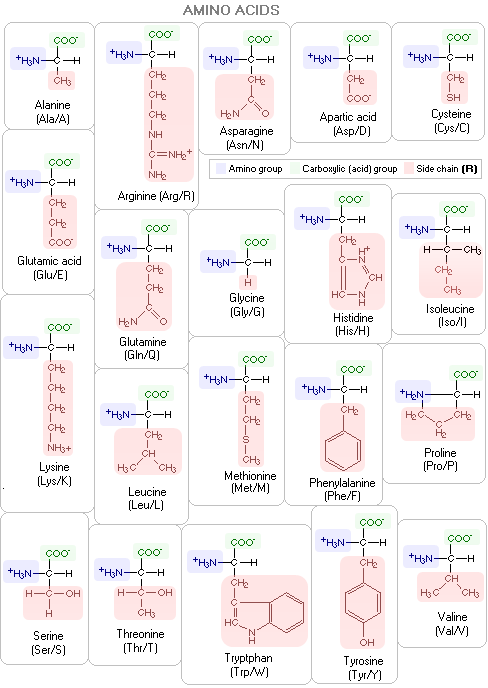
For clarity, the R groups on the above polypeptide scheme are on the same side of the backbone; actual molecules have them alternate aroundthe backbone in a periodic 3-D pattern. Below are specific molecular structures of the 20 common amino acids, with their standard three- and one-letter abbreviations. Their side chains give them different chemical properties. In addition to nitrogen, carbon, hydrogen and oxygen, two of them - methionine and cysteine - contain sulfur atoms. The carbon atom bonding the three molecular substructures - amino group, acid group and side chain - is α-carbon.
Protein can be defined as a complete, functional biological molecule build of amino acids. Its complex molecular structure, from individual amino acid residues to a complete molecule, can be organized in four main levels: 1 - PRIMARY STRUCTURE - specific order of amino-acid residues (i.e. portion of the amino acid remaining after forming the peptide bond) attached to each α-carbon, belonging to the polypeptide/protein backbone (or main chain), which consist of a series of linked hydrogen/carbon/nitrogen segments 2 - SECONDARY STRUCTURE - dominant spatial form (or backbone conformation) of a pair of protein's polypeptides, with the two most frequent forms being alpha helix and beta sheet
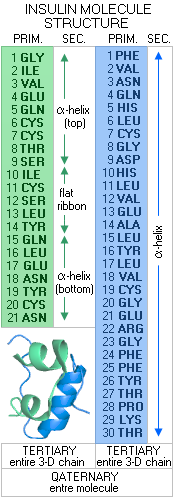
3 - TERTIARY STRUCTURE - the 3-D structure of a full-length polypeptide pair chain, and 4 - QUATERNARY STRUCTURE - the 3-D structure of the entire protein molecule, which can be anywhere from a single polypeptide to a number of polypeptide pair chains Let's look at a real protein molecule, and its structure levels. A small, comparatively very simple molecule such as that of the hormone insulin.
In its initial form, as synthesized by the DNA/RNA activity in the β-cells of the pancreas, insulin molecule consists of 110 amino acids. From cytosolic ribosomes, where it is assembled, this molecule is moved to the cell's endoplasmic reticulum, where 24 amino acids - its "signal peptide", which lead it to the endoplasmic reticulum - are removed by enzymatic action. The rest of molecule, so called pro-insulin, is then folded into its basic 3-D structure, to be transferred to the Golgi aparatus, where another signal peptide, 33 amino acids, is removed by the enzyme prohormone convertase. After two more amino acids are removed by the carboxypeptidase E enzyme, the molecule takes its final form, as shown to the left. It consists of two amino acid (peptide) chains: the A chain of 21 amino acids (green), and B chain of 30 amino acids (blue). The amino acid sequence of these chains in the primary structure of the insulin molecule. Its secondary structure is the 3-D forms of these two peptide chains. The A chain is composed of two short α-helix segments, connected with a short, nearly flat ribbon, while the B chain is a continuous α-helix. The tertiary structure is the entire 3-D peptide chain, and the quaternary structure is the 3-D structure of the entire molecule. The tertiary/quaternery structure of an actual insulin molecule also include four disulphide bridges (three between the chains, and one internal), further stabilizing the molecule. It can also combine into dimers (double molecules), or hexamers (6-molecule structure), the latter being the form in which it is stored in the pancreatic β-cells. Some of more complex protein molecules are shown below.
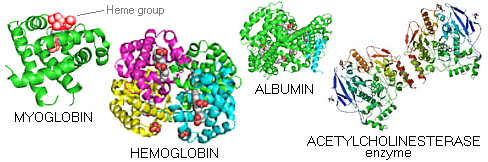
Myoglobin - a small, bright red protein very common in muscle cells, where it stores oxygen - is the first protein whose molecular structure was deciphered (Kendrew et al). It is composed of a single folded α-helix peptide chain, linked by short loops. Embedded within is the heme group - a deep red non-protein structure encasing iron atom bound to water molecule - which ties up oxygen. Hemoglobin - the red blood cell protein transporting oxygen - consists of four polypeptide chains, each having their heme group (red). Albumin is a common protein found in egg white, milk and blood plasma. Acetyl cholinesterase is the enzyme degrading one of the major neurotransmitters, acetylcholine (it contains both, α-helix and β-sheet secondary structures). This is only an incredibly small
portion of the proteins synthesized, used and degraded in the body from
moment to moment (remember that a single body cell contains some ten
BILLION protein molecules). How much of protein the body actually needs?
More about it on the following page. CONTINUES: Protein requirements YOUR BODY ┆ HEALTH RECIPE ┆ NUTRITION ┆ TOXINS ┆ SYMPTOMS |