|
■
Home ■ site map |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
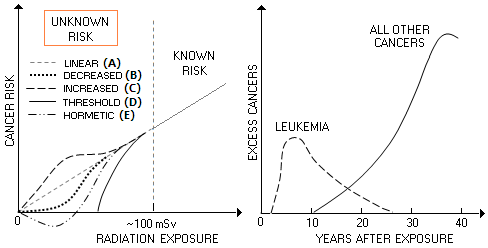
BLOG: June 2010 - December 2013 II - Mammography 18. Mammography radiation riskHow, specifically, the level of breast tissue irradiation by X-ray mammography translates into increased risk of developing cancer? The truth is - no one really knows. Statistically reliable dose-effect relationships can be established only for irradiation levels of about 100 mSv and larger. Due to the relatively low incidence and very long symptom-free (latent) period, a study large enough to identify statistically reliable rate of incidence for low-dose exposures would need a cohort that numbers from hundredths of thousands to millions, and to last decades (National Research Council, 1995). Such trial has no chance of ever being conducted, even if ethical considerations would allow it. The only remaining option is taking an educated guess of the risk induced by low-level irradiation with X-rays. There are five possible scenarios: (A) risk decreases linearly, nearly in proportion to the radiation dose (linear response), (B) it decreases at a faster rate initially, and then more slowly toward zero at zero irradiation level, with the risk lower at all points vs. linear decrease (C) it decreases more slowly initially, and then more rapidly toward zero at zero irradiation level, with the risk higher at all points vs. linear decrease (D) it decreases quickly, becoming zero at low irradiation levels ("threshold" response), and (E) it decreases quickly toward lower exposures, becoming beneficial (i.e. cancer-preventive) at very low doses (hormetic response).
Estimated risk points based on the data for solid cancers in atomic bomb survivors in Japan from 1958 to 1994 indicate the C-plot (accelerated risk below ~100mSv exposure) as the most likely shape for the low-level radiation exposure risk (Pierce and Preston, 2000). Another important information from the studies of atomic bomb survivors - many of whom were exposed to below 100 mSv doses - is that cancers caused by radiation generally start showing more than a decade after irradiation, with the peak incidence falling at nearly four decades after exposure. The only exception is leukemia, whose latent period is only 2-3 years; it reaches peak incidence within the first decade, gradually falling to near zero 25 year after exposure (above, dashed at right). However, the National Academy of Sciences, whose word is the official standard, has long opted for the linear - so called "linear-no-threshold" - risk model for low-level radiation exposures. In its latest BEIR (Biological Effects of Ionizing Radiation, 2005) report, the number of excess breast cancers per 100,000 exposed women estimated to result from a single 100 mSv (0.1 Sv, or 0.1 Gy) whole-body dose, ranges from 1171 at age 0, to 4 for exposure at 80, while the excess deaths due to breast cancer range from 274 to 2, respectively (BEIR VII, Table 12D). For premenopausal woman aged 30, the estimated risk of excess breast cancer from this radiation dose is 253 in 100,000, or 0.25%, and for postmenopausal woman aged 50 it is 0.07%. The risk of any cancer at this level of exposure is 1.1% and 0.74%, respectively.
These are the estimated risks for different cancer types, for
whole-body exposure in five selected age groups.
TABLE 2: 2005 BEIR estimates of
female cancer risk from exposure to Both, incidence and death rates due to radiation-caused cancers are significantly higher for females than males, mainly as a result of female sex organs sensitivity (also, females have more than doubled risk of radiation-caused lung cancer). For all cancers, the difference is at its greatest from age 0 (newborn) to about 30, from 86% to 55% higher, respectively. At 60, it drops to 20% higher risk (the death rates are also similarly higher for females).
For all age groups and cancers, the excess risk from a single
0.1 Gy whole-body radiation dose in the latest BEIR report is
as follows:
These new estimates are more reliable, since they are based not only on the data from atomic bomb survivors in Japan, but also data on radiation-exposed U.S. women. The uncertainty of these new figures is estimated to be ±36% which, if true, would make them quite reliable. Since the estimates are for the whole body exposure to a single 0.1Gy (equaling 100 mSv) dose, this means that every body part, including breasts, is assumed to be delivered 100 mSv of radiation. And since average mammogram, from Table 1, delivers roughly 6 mSv to the breasts, the average radiation dose to the breast from a single X-ray mammography session is about 6% of the exposure in the BEIR table.
It implies the risk
of breast cancer from a decade of average yearly mammograms
being approximately 60% of the
lifetime risk from a single 100mSv whole body exposure. The figures for several age groups are given below.
Table 3: Additional breast cancer risk from the average mammography screening test, based on BIER estimates Since the death rate is roughly 1 in 4, the added risk of dying from breast cancer is nominally about four times lower than the incidence figures (slightly lower from about age 30 down, and somewhat higher from about age 50 up). Don't forget, this is based on the average mammographic radiation dose. Women with very thick breasts can receive up to three times higher dose - or even more - so the risk for them also triples. For a woman 50y of age, that would come to some 0.12% higher breast cancer risk after 10-year period of annual mammography. This implies that about 1 in 830 women in this, most exposed group, would get breast cancer as a result of screening over 10 years. With the average mammogram at this age, 1 in 2,500 (0.04%) would get breast cancer due to screening radiation exposure over 10-year period. Considering that the expected overall rate of incidence over this period is about 3%, this would amount to about 1.3% increase (from 3 to 3.04%) in the breast cancer incidence rate due to the screening radiation. Or, out of 1,000 women diagnosed by breast cancer after annual screening from 50 to 60y of age, in about 7 it would have been caused by the exposure to screening radiation (using the 0.02 risk ratio increase for 61y of age). Which means 7 in every 1,000 BC deaths among these women would also be a consequence of the radiation from screening. Going to the 60 to 70y screening group, the accumulated radiation dose would double, but with the risk ratio increase for 71y of age less than half as large as for 61y, number of breast cancer cases due to the screening radiation would decline, to about 5 in 1,000. This decline would continue toward the older age. Limiting the scope to the typical screening population, 50-70y of age, out of 1,000 breast cancer cases, maybe 6, or so, would be due breast cancers caused by the screening radiation. If that seems to be a low number, think that this population in the U.S. numbers over 20 million. At about 0.4% BC incidence at age of 70y, we are talking about a 500 BC diagnoses, and over 100 BC deaths annually. These numbers are based on currently accepted opinion that multiple radiation exposures have nearly arithmetic cumulative effect, in which case ten mammography procedures will increase the risk tenfold with respect to the single exposure. This is probably not how it actually works; there is sufficient evidence suggesting that fractionated exposures separated in time are less detrimental than a single exposure of an equal total dose. As mentioned before, the apparent similarity is due to the offset with (officially unrecognized) greater harm caused by lower-energy X-rays. In the end, however, the figures shouldn't change significantly, because the lower actual cumulative effect would have been mainly offset by the higher (than 1) correct radiation weighting factor applied to mammography X-rays. It should be emphasized that these figures are still very approximate. Heck, the BEIR numbers for the breast cancer risk from 100 mSv exposure
are not even in agreement with the latest, What this number implies is that any given dose of radiation will inflict to the whole body approximately 1/0.12, or over eight times more cancers than to the breasts alone. In the BEIR table, this ratio varies from about four to one for the 0-30y age, to nearly seven to one for age 30-60y, and about 35 to 1 for age 60-80y. The simple average comes to about six times more cancers for the whole body, than breasts alone. That is over a third more of breast cancers than what the ICRP (International Commission on Radiological Protection) breast tissue weighting factor implies. However, it is radiation exposures before the age of 40y that matters most for the breast cancer incidence rates. Hence, the relative sensitivity of breast tissue to radiation implied by the latest BIER report is nearly twice that implied by ICRP. Just another indication of the global disarray in the field of radiology. Additional individual risk factors further increase the average risk shown in the BIER table. For instance, if you happen to be the carrier of a mutated BRCA gene, your risk of developing breast cancer due to radiation exposure may be up to several times higher (Van Leeuwen et al. 2005). Scary enough, the 20-150 mSv irradiation from 10 mammography screenings, with possible actual maximum of over 200 mSv, pretty much
puts
you in the same bag with most of the studied in the Life-Span Study (Preston et al. 2003). More than half of them had less than 50 mSv radiation exposures, and the average was about 200mSv. More so if the doses for the atomic bomb survivors are actually the nominal absorbed values, which is likely considering that the radiation weighting factor is officially identical for both, X-ray and gamma radiation. However, it is well documented that the linear energy transfer to tissues by radiation decreases as its energy level approaches and exceeds 100 keV (about the mean energy level for X-rays, and significantly higher than the usual medical X-ray level, at about 30 keV), causing the equivalent dose for nominally identical higher-energy γ-rays irradiation created by atomic bomb to be only about half as high as the nominal dose. This is probably of relatively minor importance, since it seems that the increased energy transfer is, in the case of X-ray mammography, mainly offset by the lower impact of fractionated vs. all-at-once exposure. On the danger of official assumptions: not too far back, in 1977, the United Nations Scientific Committee on the Effects of Atomic Radiation - UNSCEAR - published that radiation exposure in infancy and childhood is likely to have minimal effect on increasing breast cancer risk, due to "only a few breast cells" existing before puberty; in 1981, Gofman openly challenged this view, pointing out that those "few cells" are progenitors of all breast cells that develop after puberty - subsequent research proved that the Committee was terribly wrong. On the other hand, smoking a pack of cigarettes a day for a year irradiates your lungs with about 80 mSv effective dose, mainly from polonium 210 in tobacco. It emits α-particles (in effect, helium atom nuclei, consisting from two protons and two electrons), which are estimated to be 20 times more damaging than X-rays for given absorbed radiation dose. Due to their size and mass, α-particles are active only within short range (a few cm in air), and have very low penetrating potential (can be blocked by a sheet of paper). Thus the internal damage from smoking is caused mainly by direct contact of the isotope with lung tissues. But this is enough to cause cellular damage and initiate cancerous growth. By the way, don't be confused with the term the "effective dose for breast tissue" - that may be seen in some articles - calculated by multiplying the actual absorbed dose with the tissue weighting factor. This factor indicates an estimated relative sensitivity to radiation of different biological tissues, body parts and organs, so that the sum of weighting factors for all of them (the whole body) is 1, or 100%. Hence radiation dose X delivered to a specific organ, or tissue, gives an effective whole-body exposure that would produce this level of risk for developing cancer anywhere in the body as wX, where w is the organ/tissue weighting factor. But the wX quantity is not the effective breast dose, it is the effective whole-body dose that carries near identical estimated risk of developing (any) cancer. The effective breast dose is the absorbed dose multiplied by the X-ray weighting factor. Since this factor is officially 1, the effective breast radiation dose is nominally identical to the absorbed dose, the only difference being that the former is expressed in Sieverts (Sv), and the latter in Grays (Gy). The only use of this statistical quantity - the effective whole body dose - is to make possible relative compounding and comparison in terms of whole body irradiation. For instance, taking the usually cited average complete X-ray mammogram that irradiates breast tissue with 6 mSv, the corresponding whole body irradiation that would result in the similar risk of developing any cancer would be about 8 times smaller (since breast tissue weighing factor is 0.12), or about 0.7 mSv. Hence, if one's annual irradiation from natural sources is around 3 mSv (estimated U.S. average; the range for most of the population is 1-10 mSv), such mammogram will have it increased to 3.7 mSv, or some 24%. However, since about 2/3 (i.e. 2 mSv) of the average natural exposure comes from inhaling radon (222Rn), which is alpha-radioactive, affecting only the very top layer of the bronchial lining, and about 0.4 mSv from internal beta-decaying natural nucleotides, mainly from ingested foods, (nearly entirely Potassium-40, and very little carbon-14), affecting only the top layer of intestinal lining (beta-particles also have low penetration power), about 80% of this average dose does not affect the breasts. It is only the remaining 0.6 mSv - about 0.3 mSv from each, terrestrial (mainly radioactive Thorium and Uranium, 232Th, and 238U, respectively) and cosmic gamma-radiation - that affects the body as a whole. This 0.6 mSv whole body dose compares to the effective dose for the average mammographic screening of about 0.7 mSv. It means the chances that 6 mSv radiation absorbed by the breasts during the average mammography session will cause breast cancer are the same as those for 0.7 mSv effective, or whole body dose (from 6 mSv times the breast weighting factor of 0.12) of causing cancer anywhere in the body - and that the risk is nearly 20% higher than that coming from the 0.6 mSv whole body natural exposure, where every part of the body receives that dose. Hence, an average mammogram would more than double the effective annual dose from natural radiation affecting the breasts, from about 0.6 to 1.3 mSv. However, since these are high-energy cosmic and gamma rays which, for given dose, probably have one half, or so, of the damaging effect on tissues of X-ray radiation (officially assumed equal), the effective annual whole body exposure increase due to a single average standard X-ray mammography session is more like quadrupled: from about 0.3 to 1.3 mSv. And there's more. With the natural yearly exposure being distributed over 365 days, highly fractionated, hence much easier to handle for the cell repair mechanism, irradiation dose for natural radiation is effectively still lower relative to that from mammographic screening. Assuming, very approximately, that this makes it half as damaging as the single at-once dose of mammography X-rays, the average annual mammography would - assuming near linear risk increase with the dose - multiply breast cancer risk vs. that from natural radiation about 8 times! In other words, the risk from natural radiation is negligible in comparison. This illustrates how unrelated to the actual risk, and even misleading can be the use of the "effective dose" concept. It is merely a way of making different forms and/or body areas irradiation comparable through an estimated corresponding whole body dose. Another possible application for the effective dose is in estimating individual contribution of specific diagnostic procedures in the total annual irradiation of an average patient. But the fictitious, statistical nature of the "effective dose" has no relation to the actual cancer risk to the breast from the specific dose of radiation delivered to it. Before 2007, the breast weighing factor wasn't even applicable to the female breast cancer radiation risk. It was an averaged figure for the entire population, females and males, initially estimated by the ICRP (International Commission for Radiation Protection) to be 0.15 (1977), and revised to 0.05 in 1990, mainly as a result of broadening the list of affected body organs. The latest ICRP revision from 2007 puts its value at 0.12, nearly entirely reflecting the averaged female risk. The more specific female breast cancer weighing factor (keep in mind, it is still only a gross average) implied by the above BEIR figures (Table 2) is
anywhere from 0.25 at the age 0y to 0.05 at 60y Specifically, assuming linear risk-dose relationship, the average mammography screening test, which in all likelihood delivers around 6 mSv of radiation, increases your risk by 1/16 of the estimated BEIR risk for one time exposure to 100 mSv of radiation, for the specified age group. If you are, for instance, 40, that would be 1/16th of 141 extra cancer cases per 100,000 women, or less than 0.01%. That, of course, in the idealized scenario that you haven't been exposed to other than natural ionizing radiation before that. That is why the absorbed dose - about 6 mSv per average 2-views standard mammography screening - is the indicator of your actual risk, even if only estimated and averaged out, while the effective dose - usually given as 0.7 mSv (0.12 of the absorbed dose) - is a statistical number expressing relative risk in terms of the risk of developing any cancer as a result of whole-body exposure. Of course, mammography is not the only source of additional radiation exposure. A number of different medical diagnostic imaging procedures use some form of ionizing radiation, from regular chest X-rays to chest CAT scans and nuclear heart/lungs imaging. In fact, according to a large study published in the New England Journal of Medicine, chest irradiation from all forms of radio-imaging accounted for nearly half (45%) of the effective annual dose from medical imaging procedures (Exposure to Low-Dose Ionizing Radiation from Medical Imaging Procedures, Fazel et al. 2009), with the mammography contribution being as low as 3.1%. But, then, this figure is based on 0.4 mSv effective dose for mammography which, being calculated using the latest ICRP weighing factor of 0.12, implies 3.3 mSv averaged actual dose used. However, the actual average radiation dose per X-ray mammography session is more likely to be about twice higher, making an average contribution of this procedure to the total body irradiation by all medical imaging procedures using ionizing radiation some 6% (assuming figures for other sources correct). Since breasts do receive a significant portion of radiation with nearly all chest radiation exposures, the problem of breast irradiation and its connection to the breast cancer risk and incidence is well beyond X-ray mammography alone. The total breast irradiation from all medical diagnostic procedures may be over a dozen times higher than that from mammography alone. It implies that an average women using other radiological tests can have up to several times higher chances of developing breast cancer than from mammography screening alone. While these numbers are obviously very approximate, the data indicate that the averaged amount of radiation absorbed by breast tissue from medical radiologic tests is significantly higher than that delivered by mammography alone. And so is the breast cancer risk it creates. Considering that relatively few women uses mammography before age 40, the added breast cancer risk due to X-ray exposure from this test seems to be quite low; for women aged 40, according to Table 3, as low as 0.08% for a decade of average yearly mammographs. But in large populations the risk is not negligible: it implies 800 cases of breast cancer, and approximately 200 breast cancer deaths caused by mammography, for every million women that use the test for a decade (yes, it also implies that ten times as many breast cancers and deaths, or more, are caused by radiation from medical diagnostic procedures). In addition, the added risk can be substantially higher for vulnerable individuals. Are the benefits from mammography screening sufficient to compensate for this negative, and reach beyond - for the net benefit? More on that subject next. YOUR BODY ┆ HEALTH RECIPE ┆ NUTRITION ┆ TOXINS ┆ SYMPTOMS |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||