|
■
Home ■ site map |
|||||||||||||
|
|
Body metabolism
Body metabolism -
Cellular
metabolism
Sugars
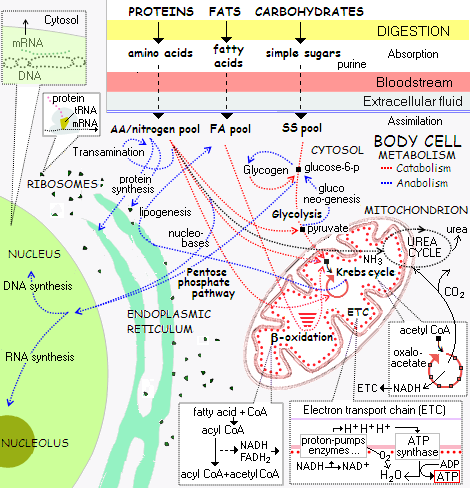
Amino acids It matters a lot how good is the food you eat, and how efficient is your digestion. But it still isn't enough to guarantee health. It is the smallest piece of the puzzle - your cellular metabolism - that ultimately determines how good you feel, and how long you live. As your cells assimilate small molecules of proteins, fats and carbohydrates broken down by digestion - i.e. amino acids, fatty acids and monosaccharides - the question of whether they will be able to use them efficiently for their - and yours - wellbeing is still unanswered. And the answer depends on the final outcome of the incredibly complex chemical interaction of billions of molecules inside each of your cells. The importance of this process is summed up in its two-word description: cellular respiration. It is literally what is keeping your cells, and your body, alive. The entire process of cellular respiration can be summarized in a single sentence. Assimilated monomers (amino and fatty acids, monosaccharides) are used either for synthesis of needed molecules (anabolism), or further degraded to free the energy needed for the synthesis (catabolism). Of course, there's much more to it. The big picture shows all catabolized monomers - fatty and amino acids, as well as simple sugars - going through a 3-step process: 1 - conversion to a simple compound called acetyl CoA, generating some adenosine triphosphate (ATP, cellular energy unit), some high-energy electron carriers (NADH, FADH2), some hydrogen ions (i.e. protons, H+) and carbon dioxide (CO2) in the process 2 - oxidation of acetyl CoA through a cyclic series of reactions in the mitochondrial matrix, called Krebs cycle (also citric acid cycle, or tricarboxylic acid cycle, TCA), which creates more high-energy electron carriers (NADH, FADH2 and GTP - guanosine triphosphate, another electron donor produced by Krebs cycle), hydrogen ions and carbon dioxide, and 3 - channeling high-energy electrons from NADH, FADH2 and GTP through a series of reactions called electron-transport chain, or oxidative phosphorylation, to create proton flow through the inner mitochondrial membrane, used to generate major influx of ATP units by ATP synthase enzyme, with oxygen enabling continuation of this process by removing (i.e. accepting) end-point electrons and then combining with protons to form water (H2O) Diagram below is the very basic illustration of cellular metabolism; that is, how cells use digested food molecules assimilated from the bloodstream in catabolic (energy-producing break-up of complex molecules) and anabolic (energy-requiring molecular synthesis) phase of their metabolism. The major metabolic pathways are summarized in the table preceding it.
*(fatty acid synthesis is initiated at sufficiently high levels of mitochondrial acetyl CoA, produced mainly from the monomers created by digestion of dietary carbohydrates and fats; excess fatty acids synthesized are bundled into triglycerides - i.e. triacylglycrol - and stored into fat tissue) There are many other metabolic pathways, both catabolic and anabolic. Very few, if any, comprise only a single type of reactions (i.e. either breaking down, or synthesis). It is the overall effect that determines the nature of the pathway. For the cell, both catabolism and anabolism are pretty much a part of one same purpose - staying alive.
The above diagram shows only the major pathways of the three generalized food metabolites absorbed at the cellular level: amino acids, fatty acids and simple sugars. In reality, the cell processes over 20 different amino acids, nearly as many fatty acids and three simple sugars: glucose, fructose and galactose. The above scheme is immensely simplified and incomplete representation of the entirety of cellular metabolism, but it is useful for grasping the big picture. In addition to the major pathways, it identifies the three central metabolites of cellular respiration: (1) glucose-6-phosphate (G6P), branching out into two major metabolic pathways, one catabolic, leading to conversion to pyruvate (glycolysis), then to acetyl CoA, Krebs cycle and electron transfer chain, while the other anabolic, leading to conversion into 5-carbon sugar molecules (pentoses) needed to build DNA and RNA structure (2) pyruvate, the end result of glycolysis, and link to acetyl CoA (3) acetyl CoA (acetyl coenzyme A), common metabolic intermediary to oxidation of glucose, fatty acids and some amino acids; its reaction with oxaloacetate produces citrate, initiating two major metabolic pathways: Krebs cycle and fatty acid synthesis Behind this big picture, there are many smaller, auxiliary and minor metabolic pathways, catalyzed by thousands of enzymes. Many of these pathways partly overlap, or have one or more alternative pathways, which are activated if the preferred one is inefficient or not functioning. This, in turn, changes the overall metabolic outcome, possibly significantly. Many pathways do interfere with other pathways to some extent. Also, many reactions are reversible, so the cell can switch metabolic paths back and forth, according to its needs. An actual image of the cellular metabolism is a highly coordinated chaos of billions of molecules and sub-molecular entities continuously interacting both, within the cell and in its immediate surroundings. To make it all possible, there has to be sufficient energy available wherever it is needed. In general, degradation (catabolism) of disposable complex molecules into simpler molecules provides the energy for synthesis (anabolism) of more complex molecules needed by the cell. Any disturbance in the efficacy of major catabolic pathways - glycolysis, β-oxidation and amino acid degradation fueling glycolysis or Krebs cycle, or electron transport chain - will negatively affect cell's viability. A common cause of such disturbances are inhibitions in the cell's enzymatic activity by environmental toxins, oxidative damage and nutritional deficiencies. The enzymes are the lifeline of cellular respiration; if they are damaged or incapacitated, cellular processes that they carry dwindle. It can be anything from splitting the molecules for energy, to the synthesis of cellular structures. As a result of inefficient enzymatic action, cells become less viable, sluggish, and more vulnerable to further damage; at worst, they die prematurely, or disassociate from the body, possibly turning into some form of malignant growth. If significant in the extent and duration, such obstructions of cellular metabolism will manifest as various symptoms of compromised health and/or degenerative diseases. In other words, all the good, and the bad happening to your health comes from this tiny microcosm - the body cell. This warrants a bit closer look into cellular metabolic pathways for each of the three major macronutrients. Let's start with the sweet stuff: the sugar metabolism. YOUR BODY ┆ HEALTH RECIPE ┆ NUTRITION ┆ TOXINS ┆ SYMPTOMS |
||||||||||||