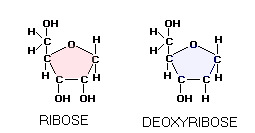|
■
Home ■ site map |
|
Carbohydrate intake and body functionWhile not nearly as versatile in body functions as fats and proteins, carbohydrates are that main source of energy making it all possible. As their name implies, they are made of carbon and hydrogen. Despite being very simple in their basic structure, dietary carbohydrates do have markedly different properties in each of their four main forms: ▪ simple sugars ( monosaccharides)▪ double sugars (disaccharides) ▪ starches (oligosaccharides or polysaccharides), and ▪ cellulose Simple sugars like glucose and fructose (fruit sugar) are nearly instant source of energy; immediately absorbed into the bloodstream, they are readily available for assimilation by body cells and use in their energy producing cycle. Double sugars, the very familiar example of being sucrose (table sugar), are built of two simple sugars - such as glucose and fructose in the case of sucrose - joined together. In the intestine, they are quickly broken down into simple sugars. Starches are complex carbohydrates found in grains, potato and so called "starchy" vegetables. Their single molecule is built of many - sometimes thousands - glucose molecules. For that reason, it takes generally a longer time for the body to break them down to glucose. That results in extended, more even glucose assimilation - a healthful alternative to glucose rush caused by most simple and double sugars.Cellulose is, just like starches, composed of many glucose molecules - but a different form of glucose molecule. As a result, cellulose molecule has much stronger bonds. While starches are used by plants for storing energy, cellulose is used to build their supportive and protective structures. Cellulose is the main component of dietary fiber, food component not significant as an energy source, but essential for health. Molecular structure Why is cellulose so much different than starches, despite both being built of the same material - glucose? Answer to that lies in the molecular structure of glucose. All carbohydrates are built of carbon, hydrogen and oxygen atoms. Simple sugars have one sugar unit - a structure consisting of carbon skeleton, with one atom of oxygen and two atoms of hydrogen for every carbon atom - per molecule. The most common simple sugars have five or six carbon atoms, thus are called pentose and hexose, respectively.
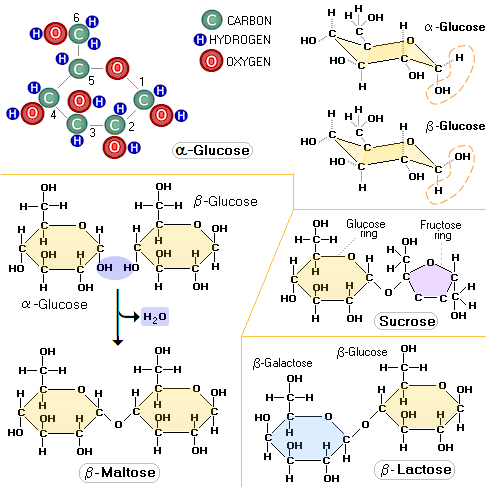
The two most famous pentose sugars are ribose and deoxyribose, used by the body cells for building their RNA and DNA molecules. Inside the cell, both sugars are synthesized from glucose in a series of chemical reactions known as pentose phosphate pathway, one of the major cellular metabolic pathways in the human body. Two simple sugars bond together, forming a double sugar, as a result of condensation reaction, in which they have a water molecule removed. The table sugar, sucrose, is built of two simple sugars: glucose (hexose) and fructose (pentose). Lactose, the milk sugar, is built of two hexose sugars, glucose and galactose, and so on.
Glucose molecule has two distinctive forms, or
isomers: α-glucose and
β-glucose. The only difference between them is in the spatial
orientation of their hydrogen atom (H) and hydroxyl group (OH) bound to their carbon 1
atom (carbon atoms in sugar molecules are assigned specific numbers).
It is above the plane of the ring in α-glucose, and below it in
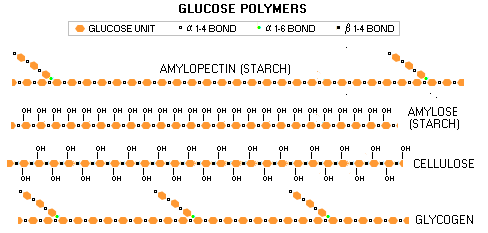
Large carbohydrate molecules - glucose polymers - like starches, and their animal-tissue analog, glycogen, are formed in the same manner as disaccharides. Structurally, the main difference is that they have many more glucose molecules linked together. However, bonding in chains also gives them different chemical and dietary properties.
While glucose and most disaccharides are water soluble and hydrophilic (attracting water, making them bulky), starches are not soluble, which makes them more suitable as a storage form. Having many more linked molecules also slows down digestion of starches, which is done by hydrolysis (adding water molecule). The enzymes degrading starches to simple sugars, amylases (from amylum for starch), are present in the saliva and also secreted by the pancreas. Being bound in long chains also deprives starches from the sweet sugary taste. This is due to different 3-D arrangement of their OH groups: they don't fit into the sweet receptors on the tongue, as those of simple sugars do. Body function Body converts all carbohydrates - except cellulose - into body's preferred form of energy, glucose (blood sugar). The excess of carbohydrates not needed for the energy body converts into fat (triglycerides) and stores it primarily in so called fat cells. Both, simple and double sugars burn quickly giving near-instant burst of energy, while starches burn more slowly, providing longer lasting, more stable energy source. Every gram of carbohydrates burned for energy releases about 4 calories on average (sugars 3.7 and starches 4.2 calories per gram). However, carbohydrates play much more complex role in the body than being just a plain energy source. On the cellular level, they combine with proteins, forming glycoproteins, and with lipids, forming glycolipids, a large group of compounds needed for many vital body functions (prefix glyco- originates from the Greek word glukus, for "sweet"). The sugar group (specific forms of glucose, galactose, fucose, manose, xylose, etc.) in glycoproteins is needed for proper structuring and function of these important cellular proteins. Glycoproteins have wide variety of functions, from the immune system functioning (antibodies, major histocompatibility complex), to proper platelet function, egg-sperm interaction, or connective tissue health. Some glycoproteins have hormonal functions, such as follicle-stimulating hormone, or TSH (thyroid-stimulating hormone). Glycolipids are crucial for proper functioning of the cellular membrane in substance exchange, as well as for inter-cellular interactions, such as tissue building. Another important structural function of sugars is in our very genetic material, namely helicoidal DNA and RNA strands, which are built fro phosphates and monosaccharides deoxyribose (DNA) and ribose (RNA). The official U.S. adult dietary intake recommendation (DRI, for "Dietary Reference Intakes") for total carbohydrates is 45% to 65% of the caloric intake. A simple math shows that it doesn't fit well into the government's own "acceptable" ranges proposed for macro nutrients, since that for fats is 20% to 35% and for proteins 10% to 35%. In other words, if you are close to the "acceptable" maximum of 35% with both, fats and proteins, you are left with only 30% or little more for carbohydrates. Although it still can be labeled officially "acceptable" according to the DRI, such diet would be very unhealthy for most people. Since 35% of the calories is, in fact, excessive for either fats and proteins, why not simply make their respective general recommendations max out at 30% and 25%? It is both, healthier and adds up to a proper sum with the minimum recommended carbohydrate intake of 45% of the calories. Prevailing educated - should we also say impartial -
opinion is that the low end of the DRI range for both, fats and
proteins - 20% and 15% respectively - is actually closer to their optimum intake level for most healthy
adults. Thus carbohydrates optimally should make about
65% of
the total caloric intake. Most of them should be complex carbohydrates;
any significant intake of simple carbohydrates (sugars) is likely to
have negative
short- and long-term health consequences. Sugar bluesHigh intake of fast burning simple and double sugars causes surge in blood glucose level, which stimulates the pancreas to start excreting insulin in an attempt to keep blood glucose at the safe level. Significant amount of sugar will likely provoke enough of the insulin kick to push blood glucose level down too much, creating sudden low-blood-sugar condition. Body's response to it is feeling of hunger, tiredness, and need for instant energy. That instant energy is, of course, sugar. The feeling of hunger it produces also stimulates over-consumption of other foods, both leading to obesity. In addition, excess sugar deprives you of - among other nutrients - chromium, which makes you crave sugar even more. For sweets lovers, it spells "Welcome to the vicious circle!" If excessive sugar intake comes from consuming nutritionally inferior foods - which is frequently the case - the body is literally starved by the luck of nutrients, particularly vitamin B complex, needed to metabolize sugars. Since the metabolism is body's first priority, it will get needed B vitamins at any price, even if it takes stealing them from where they are most abundant: your brain. It can affect your mood and other items in your "head department" in unpredictable - but likely negative - ways. Excessive consumption of simple and double sugars puts additional strain on the pancreas and its insulin function, which with time can lead into diabetes type 2. Overeating and obesity, nutritional deficiencies and acidosis (unhealthy high level of blood and cellular acidity) often accompany sugar over-consumption. Needless to say, any of these make the body vulnerable to further health erosion. Research indicates that chronically elevated glucose level can alter body's metabolic mode by affecting gene expression. The elevated-glucose mode makes the body less resistant to oxidative damage and to external threats in general. Longer term, it may significantly shorten lifespan. It is not only sugars that can negatively affect body metabolism, both short and long term, by rising blood glucose level. In general, all carbohydrate-rich foods have that potential, usually commensurate with their sugar content. This is not a reason to avoid all carbohydrates - we do need them to live - just a reminder that any dietary excess is likely to result in compromised health. With respect to glucose metabolism, of particular concern are highly processed carbohydrate-rich foods, which can be nearly as bad as sugars, as their glycemic index and glycemic load values show. There is no official recommendation for
simple-to-complex carbohydrates proportion in the diet, although general consensus is that
complex carbohydrates are preferable, and ought to make about
90% of
the carbohydrate intake. On the average, Americans consume nearly half and half
of each, sugars and starches. Cellulose and dietary fiberCellulose is different from sugars and starches in that its molecules are more rigid, making it indigestible to humans (and nearly all mammals); therefore, it doesn't contribute caloric value, but it does provide needed bulk for proper functioning of the digestive tract. Inversely, lack of dietary fiber promotes constipation. Dietary fiber also slows intestinal absorption of glucose, which has stabilizing effect on blood glucose level. Cellulose is the main component of dietary fiber (others include lignin, dextrin, pectins, beta-glucans, oligosaccharides, etc.), which can be insoluble and soluble. Insoluble fiber - usually found in plant's skin, or shell (husk) - absorbs fluid, forming bulk needed for efficient elimination, also helping in the removal of toxins from the intestines. In the absence of fiber, toxins are reabsorbed into the bloodstream at a significantly higher rate. Soluble fiber, found in the inner portion of plants - dissolves in the intestinal fluid and ferments in the colon. This produces short-chain fatty acids (along with colonic gasses) - in particular butyric acid - which are reabsorbed through the colonic wall and play significant role in assisting pancreas in stabilizing blood sugar. These fatty acids also have a number of other beneficial health effects: stimulating immune system, proliferation of friendly colonic bacteria, suppressing production of LDL cholesterol and triglycerides, and others.7.Presence of fermentable fiber also seem to improve absorption of minerals, especially calcium. Dietary fiber is found only in foods of plant origin, with most unprocessed plant foods being a good source. However, due to relatively high consumption of processed and animal-origin foods, it is alarmingly low in the typical U.S. diet. Average intake of dietary fiber in the U.S. is below 50% of the recommended 20g-35g daily adult intake. YOUR BODY ┆ HEALTH RECIPE ┆ NUTRITION ┆ TOXINS ┆ SYMPTOMS |