|
■
Home ■ site map |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
BLOG: June 2010 - December 2013 I - Breast cancer risk factors 9. Factors initiating breast cancer: RadiationWhat evidence points to radiation exposure as a significant - or the most significant - factor that directly causes breast cancer (BC)? Not long ago, it's been thought that the only dangerous form of radiation is so called ionizing radiation, from ultraviolet, through X-rays to gamma (nuclear) rays. The link between nuclear radiation and breast cancer incidence is well established: breast cancer incidence is consistently higher in areas with increased exposure to radioactive materials, from the post-war Japan, to the U.S. counties situated within 100 miles from nuclear reactors sites. But this is not where radiation exposures stops. Low-level exposures to electrical power field (from power lines, appliances, dirty electricity) seem to be also increasing the risk. Mortality from breast cancer is about 40% higher among female electrical workers (Loomis et al. 1994). By interfering with body's extremely delicate cellular circuitry, even the standard low-level exposures can alter cellular functions, including gene expression, mechanisms suppressing cancerous growth, or hormonal functions (specifically, they may suppress melatonin production, resulting in higher levels of estrogen and prolactin). Harmful effect of mobile phone radiation cannot be ruled out as well. However, ionizing radiation from medical diagnostic procedures is the most significant form of radiation potentially causing breast cancer. Its high energy levels can damage DNA structures either directly, or by initiating chain formation of highly reactive particles within cell. High energies associated with ionizing radiation inflict DNA damage that is harder, or much more hard for the cell to repair. For instance, estimates are that the repair efficiency of a single DNA strand break is 99.99%, while only 90%, or 1000 times lower, for the double strand break, which is the kind commonly inflicted by ionizing radiation. Low-dose X rays, as well as higher-dose CAT-scan nuclear medicine exposures, routinely used in the medical diagnostic field, are the main source of ionizing radiation. One of these X-ray exposure sources is, of course, mammography (note that most sources tend to show minimum radiation estimate for medical diagnostic procedures, sometimes deceptively low, as is often the case with mammography). According to John W. Gofman, M.D. Ph.D. (medical doctor and nuclear physicist, 1918-2007), the late Professor Emeritus in Molecular and Cell Biology at the University of California at Berkeley, radiation expert and long time advocate for more judicious use of ionizing radiation for medical diagnostics, radiation exposure probably causes as much as 75% of all breast cancers. Gofman's estimate is based on his meticulous analysis of available evidence (Preventing breast cancer, 1996). As he explains, this does not mean that all other factors together cause the rest of 25% breast cancers. Rather, since nearly every breast cancer is a multifactorial disease, that radiation is among its major causes in 3 out of 4 women with the disease. Since all of these major factors are normally needed for the final outcome, this means that without the radiation factor breast cancer rate would be cut by nearly as much, down to about a quarter of its current level. To clarify how cancer becomes a disease, i.e. symptomatic, we should understand that initiation of cancerous cell transformation by either radiation, chemical carcinogen or virus is the necessary condition for the formation of malignant cells, but does not necessarily suffice for it to become symptomatic. These cancer-initiating factors cause cancerous cell formation at numerous body locations throughout lifetime in most, if nor all people. But it is up to the interplay of all (known and unknown) predisposing and cancer-promoting factors
whether it will progress, or not, and if it does how long it
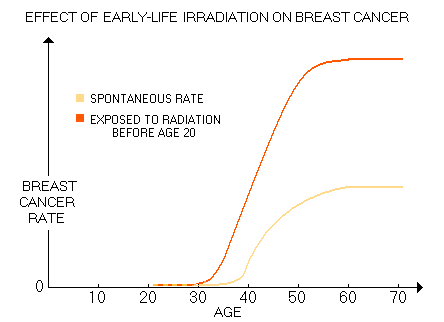
will take For many, radiation exposures span from mother's womb to adult years' diagnostic procedures. Early exposures are the riskiest, but the effect of irradiations is cumulative, thus those latter in life also add to the risk. Considering that for a cancer to develop, multiple alterations of the DNA - up to 10, or so - are needed, damage to chromosomes caused by every additional exposure to ionizing radiation (gene deletions, translocations, double DNA strand breaks, etc.) increases the chances for it to take place. The more so for those already predisposed to it by inherited adverse genetic polymorphism. Based on the data for 12,000 Japanese females exposed to atomic bomb radiation below age 20 (average 10, based on Land 1993 and Tokunaga 1994), Gofman came to this generic plot of their breast cancer rate increase vs. spontaneous rate:
This risk is significantly lower for women exposed to radiation after age 20. Gofman's 15-year old assessment is in good agreement with the latest BEIR report, which sets the radiation risk at age 10y as five times higher than at age 40y. The reason seems obvious: the rate of cell multiplication is significantly higher at a younger age, while defense mechanisms, like body's oxidative protection and DNA repair are still immature. The result is significantly higher breast cancer incidence in general, and especially the incidence of early breast cancer. The most vulnerable population are women with genotypes already compromising DNA repair efficiency or other cancer-suppressing mechanisms, particularly when combined with the presence of factors accelerating the rate of cellular division, such as elevated levels of estrogen, estrogen mimics, IGF and/or insulin. In other words, while given radiation dose will induce similar level of damage to DNA in any individual woman, whether it will progress into a symptomatic cancer or not, and how fast it will spread depends on the specific interaction of the multitude of BC predisposing and promoting risk factors. Considering Gofman's qualifications and experience, his estimate of up to 3 in 4 breast cancers being caused by medical diagnostic irradiation should not be discounted. Not necessarily as a correct figure, but as the well founded gross indicator of the magnitude of this causative factor in the overall incidence of breast cancer. According to a recent study (Exposure to Low-Dose Ionizing Radiation from Medical Imaging Procedures, Fazel et al. 2009, with nearly million subjects and over 3.4 million procedures), the mean annual radiation dose (effective dose) due to diagnostic imaging ranged from 1.2 mSv to 4.9 mSv for women aged 18-34y to 60-64y, respectively. Mean annual dose was 2.4 mSv (somewhat lower than the population average, due to exclusion of the 64y+ group), with the annual effective dose dose due to mammography alone of only 0.074 mSv. However, since it was calculated for the entire study population, roughly half of which were male, for female alone should be about twice higher, or 0.15 mSv. And since more than a half of female population does not do screening mammography, for the screened population it comes to about 0.4 mSv a year (that is, in fact, the value of average mammography-delivered effective dose assigned to mammography by authors, based on their sources). Now, about 45% of the total annual dose in the study came from various forms of chest procedures. Knowing that most of torso procedures not centered at the chest also contribute some portion of radiation to the chest area, we can safely assume that more than half of the total effective dose does fall onto the chest.
Hence those 0.4 mSv of radiation from
mammography alone compares to anywhere from 2 to 3.5 mSv, or so, total
chest radiation exposure for the population 50y of age and above
(below is study data for the total annual effective dose for all
study groups).
In other words, the average total chest radiation delivered to these women is 5-9 times the radiation delivered by mammography alone. The more realistic average effective dose for mammography is higher, about 0.7 mSv, but it is likely that the doses for other procedures in the study were generally underrated as well, roughly offsetting for the lower mammography dose in relative terms. The reason is that the study uses effective doses as quoted in Mettler et al. (Effective Doses in Radiology and Diagnostic Nuclear Medicine, 2008). Most of the procedures/doses from this study are shown below, for information as well as for illustration of the extent of medical use of ionizing radiation.
These are more or less in line with the usually cited doses, but how much we can trust them? The more radiation risk becomes known to the public, the more the official medicine tries to downplay medical radiation exposure. So, Mettler et al. plainly states that radiation doses from radiologic and nuclear medicine procedures - up to 70 mSv averaged single dose - are comparable to 3 mSv average yearly natural exposure. How so? Just for being highly fractionated - in effect continuous - natural dose acts as roughly a half lower single dose, and that is comparable to up to a hundredfold higher doses from multiple medical procedures? How do these doses compare to the national safe occupational exposure limit - 50 mSv annually for nuclear workers, less than half as much for administrative jobs - or 1 mSv annual limit for public exposure? How do those compare to the level of irradiation of the Hiroshima/Nagasaki survivors study, where 41% of them averaged 1mSv of bomb radiation, 32% averaged 19 mSv, 5% averaged 53mSv, 16% averaged 146 mSv, and 742 and 1970 mSv averaged 3% each? In other words, 94% of the survivors
were exposed to doses and nearly half of them to significantly lower doses. How do they compare to Gofman's finding, supported by the evidence from nine (of other researchers') studies, that no so called "low dose" of ionizing radiation - including as low as 1 mSv - is safe with respect to causing excess cancers? These few questions help understand how biased, misleading and arrogant is the statement by Mettler et al. which is fairly in line with what the official medicine is promoting. Usually cited medical diagnostic doses have downward bias due to several reasons. The most important one is that they are generally based on the "catalog doses", assuming near-optimum use, handling and functioning of the imaging equipment. This is seldom the case in the real world, with the actual doses varying greatly not only from one institution to another, but within one same institution as well. Doses tend to be higher simply because suboptimal image processing (underprocessing, film or digital) requires higher doses to at least partly compensate for. For instance, the 1989 NCRP Report states that (mis)adjusting digital radiologic systems to provide more shades of gray than needed, increases the dose by 5 to 10 fold over the optimum (Gofman fought for correcting and standardizing radiation procedures, pointing at both, danger of radiation and great potential for minimizing the radiation dose). Also, often times it is only the single image dose that is quoted, even when multiple images are routinely taken. For instance, cranial CT average for multiple scans is 50 mSv, 2-minute barium contrast G-I fluoroscopy 80 mSv, and so on. Part of the bias is that the official dose often does not include all irradiated organs. One drastic example are the most common form of chest X-rays. The commonly cited effective dose for the standard (posteroanterior) chest X-rays - by far the most frequent procedure - is 0.02 mSv, while the actual dose is more likely to be closer to 0.1 mSv. All considered, the actual cumulative dose from diagnostic radiation is very likely higher, possibly significantly so, than one obtained from the "book values" and assumed near-optimum doses. Considering that most women in the study (79%, vs. 58% for males) did have one or more radiation procedures, and that significant portion of women - from 12% for 18-34y to 39% for 60-64y group - received moderate-to-high radiation doses (3 to 50+ mSv annual total, implying nearly 2 to 30+ mSv chest dose), quite a few women received chest radiation dose many times that of the standard average mammography screening of about 0.7 mSv (more so considering that the study, relying on commonly published data, generally underestimates radiation dose). While the portion of chest-delivered radiation absorbed by the breasts vary with the procedure, it is commonly significant, often in the 1/3 to 1/2 range. This means that in the 50y+ population nearly 40% of women receives additional breast radiation anywhere from the level of average X-ray mammography to a dozen times, or more, higher. And we know that even mammography dose, while acceptably low for most women, can present unacceptable risk for those highly vulnerable. And that radiation effect accumulates with every dose received. From another angle, the highest quartile, or about 10% of the women, may have received up to a few dozen times more radiation to the breast than from one standard mammography. If we add up all mean annual radiation doses from diagnostic tests between age of 18 and 59y, and take the average, it comes to 96 mSv accumulated effective dose. Assuming, again, very approximately, more than half of it going to the chest area, and more than a third of that to the breasts, gives about a quarter of it, or 24 mSv, being absorbed by the breasts (again, if we go to the highest quartile (this time 25% of all women) the dose would significantly higher). That is more than three times higher effective dose than the 7 mSv accumulated from 10 average annual mammograms between the age of 50y and 60y. Thus, based on the nominal dose, it would add proportionally more breast cancer cases - and deaths - at the age of 61y, i.e. 24 per 1,000, for the total of 31 per 1,000. But this number is flawed in more than one way. First, as mentioned, the published doses used for computation in the study have downward bias. The actual doses are certainly higher. Second, accumulated diagnostic radiation from 0-18y period is not included. In fact, we should put from -0.75 to 18y period, since fetal irradiations are rather common, and not negligible. It takes place during diagnostic, therapeutic and interventional radiation procedures on pregnant women. Also, irradiation of the neonates is rather common, and can be quite intensive, particularly for premature and congenitally challenged infants. Third, and most important: plain numerical average of absorbed doses greatly underrates the risk of pediatric and neonatal irradiation. For any given radiation dose, risk ratio increases exponentially toward younger populations. According to a recent study (Use of Medical Imaging Procedures With Ionizing Radiation in Children, Dorfman et all. 2011), about half of all U.S. females 18y and younger are exposed to medical diagnostic radiation (table below).
The study did not attempt to give estimates of irradiation dose, "because methods for accurately quantifying the effective dose of ionizing radiation exposure (i.e. its detrimental biological effect) in children are controversial", which sounds more like an excuse for not to have it included. It is certainly possible to give an estimated likely minimum-maximum range, based on the International Atomic Energy Agency's "controversial" RPOP (Radiation Protection of Patients) data. It tells a lot that children are given this magnitude of diagnostic radiation without actually knowing "accurately" what effective dose of radiation they are exposed to, doesn't it? But let's go back to its role with respect to breast cancer risk. Study data indicates that the frequency of all radiologic procedures from 0-18y of age (w/o neonatal and fetal irradiations) is comparable to that in the 18-34y group from Fazel et al. with probably somewhat lower proportion of high-dose procedures. Also, that the 10-18y group has more procedures than 0-2y group, which in turn has more procedures than 2-10y group. The largest difference is for multiple CT and nuclear medicine imaging (but not fluoroscopy), where the 10-18y group has up to several times more procedures. It suggests that this group has higher exposure than the rest, possibly significantly, but there is no specific data. In all, mean total radiation exposure from medical diagnostics in
the Assuming that the actual total dose is 30% higher than that based on the "book values" - which is probably conservative - would, with 16% increase from including 0-18y age range, produce 50% higher total effective dose than 96 mSv, i.e. 144 mSv. Accordingly, breast cancer incidence and deaths due to irradiation at age 61 would come to 36 in 1,000 without 10-year annual mammography,
But the most important factor is yet to come. So far, we were assuming flat radiation sensitivity for all age groups, using the 60y sensitivity figure to get the added breast cancers due to mammography radiation, as well as to add those due to diagnostic radiation, in proportion to the dose. This, however, neglects that sensitivity to radiation - i.e. cancer risk - increases exponentially toward the younger age. And a glance at the BEIR VII cancer risk estimates reveals that, for a given dose, breast cancer risk for an infant is nearly 40 times higher than for 61y old. To factor in this great variation in sensitivity, we'll take the arithmetic sensitivity average from the BEIR VII table for all age groups, derive the ratio for the 61y old sensitivity, and multiply it with the number of radiation-induced cancer of 36 in 1,000 (w/o mammography) obtained with flat sensitivity rate for the lifetime accumulated radiation dose. This, of course, is neither proper, nor terribly accurate, but it's simple and will serve the purpose of creating a big picture indicating the possible - and probable - magnitude of radiation as a breast cancer causative factor. With the sensitivity-based incidence average for all age groups of 498, the ratio vs. 61y old incidence is 16, and radiation-induced breast cancers at age of 61y explode to 576 in 1000. Adding those seven due to the radiation from 10-year annual mammography will only raise it to 583. So, we've come to 58% of all breast cancers as radiation-induced - not far from Gofman's 75% estimate. Of course, the numerical similarity is accidental in that Gofman came to his number with a much more accurate approach. But, on the other hand, it shouldn't be accidental since our calculation, as approximate as it is, is still based on the valid gross parameters. One thing that Gofman did consider, and we didn't, is that the levels of diagnostic - and mammography - radiation used here are different than in the time past, and so is the manner in which radiation is used. It wasn't too long ago, in the early days of radiography during the first few decades of the 20th century, that there was no agreed upon dose limit for X-rays. It went from using erythema (reddening of the skin caused by radiation in the 3-4 Sv range - yes, 3,000-4,000 mSv) as the "stop" indicator, to setting the limit to 1 Sv in New York hospitals in the late 30s. For comparison, a single 3-4 Sv whole body dose can be deadly (LD50 whole body dose for X-rays is 5 Sv). The main reason for these enormous doses of radiation were low quality films, requiring long exposures, up to an hour, or more. It was, of course, practiced only because it wasn't known at the time how harmful to the body is ionizing radiation. The doses kept shrinking with technological advance, but nothing happened quickly, including learning more about ill effects of radiation. Excessive use of medical irradiation in addition to high doses included unnecessary wide fields, irradiating organs and body parts that didn't need to be irradiated, unnecessary multiple procedures, as well as indiscriminate use of radiation for a host of non-malignant diseases and ailments. The youngest were not spared either. For instance, some pediatricians were doing monthly fluoroscopy on asymptomatic infants and young children for screening purposes. The amount of irradiation of these children was enormous. X-ray pelvimetry, irradiating both mother and fetus, were common, and so on. It was only in the 1960s that radiology took decisive turn toward setting more strict standards and control of the use of radiation for medical purposes. Why is this important? Because the majority of screening population from the mammography era - from the early 1980s to this day - which is approximately women 50-75y of age, was exposed to the
medical radiation regime from the 1960 The official spin is that medical radiation doses have fallen substantially since, but it doesn't say much about change in the level of total irradiation. While we can say that the doses, as well as indiscriminate use of radiation, are significantly reduced (part of it is abandoning chest X-rays for TB screening, which delivered much higher doses than today, up to 100-fold in mobile units), other factors - higher number of radiologic procedures per 1,000, lower doses per image with digital radiography partly offset by more images taken, emergence of computed tomography (CT), nuclear medicine and interventional radiology, all of which tend to deliver high doses - precipitated its expanded use. If the total dose is lower these days, this directly implies that the figure of cancer incidence obtained based on it will be underestimate, since most of women were exposed to higher doses in the past. While the consensus seems to be that radiation exposure from medical procedures is lower nowadays than what it was a few decades ago, and before, no one can tell by how much. The reason is that no one can tell what was the actual volume of medical radiation procedures back then. Gofman allows that non-recorded doses from that time may approach, or even exceed those on record. Situation is better these days, but we're still far from having an accurate picture. Basically, we deal with the gross estimates of the volumes for
the major types of For instance, UNSCEAR (the United Nations Scientific Committee on the Effects of Atomic Radiation) states that the estimated number of diagnostic X-rays in the 1985-1990 in the U.S. - approximately 200 million, excluding 100 million dental X-ray examinations and 6.8 million diagnostic nuclear medicine examinations - "could be an underestimate by up to 60%". And that only adds to already mentioned bias from assuming near-optimum (minimum) doses when calculating total exposure. And it is less important by how much is the total dose today smaller than before - if it is. What matters is that it is still high enough to remain the major direct causative factor in most cancers,
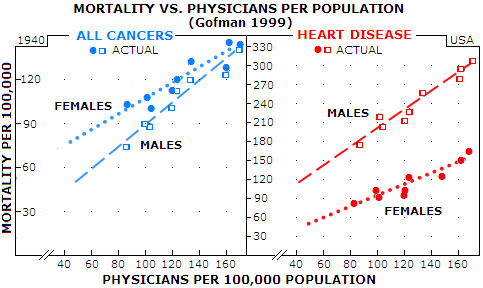
But we will continue to hear the officials and medical establishment calling it "spontaneous" cancer rate, the hype perpetuated for the purpose of hiding what has been done to the people through officially condoned medical practices, and for dwarfing the estimated cancer risk of itemized radiologic procedures. What is spontaneous about getting cancer induced by medical irradiation, or by chemical carcinogens dropped into our air, food and water? The only spontaneous, or natural in origin cancer could be coming from viral infections, and those are probably less than one tenth of the total. Responding to a 1976 study reporting inverse relationship between the level of natural background radiation and cancer rate in the U.S. (Frigerio and Stowe, one of many attempts to present the "evidence" for low radiation doses - such as those from medical imaging - being actually beneficial, not damaging), Gofman proved their conclusion baseless. But he went beyond that, analyzed other data from their study, and found a very surprising, shocking, indeed - or not really - strong actual correlation: one between the cancer rate and number of
physicians Graphs below illustrate how strong is this correlation based on official 1940 data for the U.S. Data points are grouped so tightly around the interpolated line that there is very little chance it is accidental. As Gofman said, such correlations demand an explanation - especially when it is a matter of life and death - literally. He analyzed the data up to 1990 (Radiation from Medical Procedures in the Pathogenesis of Cancer and Ischemic Heart Disease: Dose-Response Studies with Physicians per 100,000 Population), and strong correlation held on. There is as strong correlation for ischemic heart disease too, while for all other diseases (including female genital cancers) the correlation is negative, i.e. more physicians result in lower mortality rate.
In all cases shown, the trendline slope is narrowly determined by the actual data point, indicating strong linear positive dose-response relationship between the increment of increase in physicians-per-population and that in the death rate. Should it be surprising, knowing that one gets radiation treatment through her/his doctor, and that radiation is a known initiating factor of all cancers? As for heart diseases, Gofman proposed the mechanism by which radiation causes degradation of smooth muscle cells - by producing dysfunctional mutated cell clones - of the coronary arteries (he was among the first, if not the very first to point at radiation being a causative factor in heart disease). By projecting interpolated lines backwards to the zero physicians level, we can get an indication of the true spontaneous mortality rate - or, more accurately, the physician-free rate. For cancers, it is nearly 2 and 10 times lower for females and males, respectively, while 20 and 5 times lower for ischemic heart disease, in the same order. In other words, in the hypothetical area w/o physicians, females would have about a dozen times lower heart disease rate, and several times higher cancer rate than males. That discrepancy remind us that there are factors other than radiation influencing death rates. For heart diseases, part of the explanation is that females here are naturally better protected due to female hormones. On the other side, in 1940 female genital cancers were outnumbering the only significant male cancer - prostate cancer - by nearly threefold. More importantly, they are largely independent of medical radiation exposure - in fact, as mentioned, it is the only group of cancers that has negative correlation with the number of physicians per population (i.e. the death rate decreases with the increase in the number of physicians). Putting it all together, there is more than enough evidence indicating that medical radiation is a significant - probably the
most significant - causative factor in the majority of cancer forms -
Radiation dose accumulated from medical procedures using ionizing radiation dwarfs radiation from mammography screening, fueling cancer epidemics ever since the beginning of the 20th century. Without it, chances are, more than half of breast cancers wouldn't have happened. But medical radiation is still only one of the three breast cancer initiating factors. The other two are: (1) chemical carcinogens, and (2) viral infections. The latter is probably the smallest in magnitude, but can not be assumed insignificant. YOUR BODY ┆ HEALTH RECIPE ┆ NUTRITION ┆ TOXINS ┆ SYMPTOMS |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||